

Extralobar sequestration | Intralobar sequestration | |
|---|---|---|
Site | External to the lungs | Within the lungs |
Present in | Infants | Older children |
Cause |
|
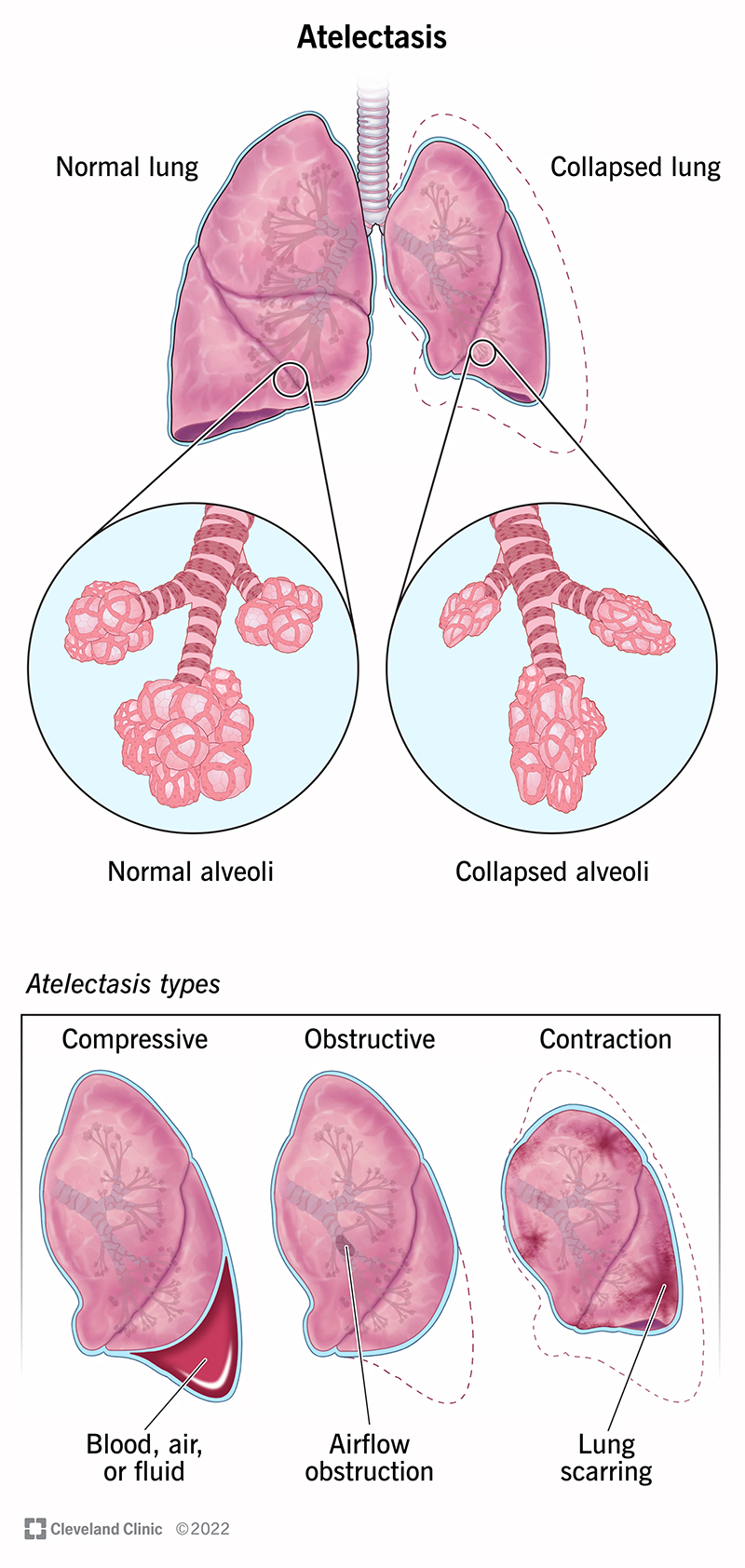
Resorption (Obstruction) atelectasis | Compression atelectasis | Contraction atelectasis | |
|---|---|---|---|
Cause | Complete obstruction of an airway caused by
| Accumulation of significant volumes of
| Focal or generalized pulmonary or pleural fibrosis |
Mediastinal shift | Towards the atelectatic lung | Away from the affected lung |
Obstructive lung disease | Restrictive lung disease | |
|---|---|---|
Characteristics | Increase in resistance to airflow due to partial or complete obstruction at any level from the trachea and large bronchi to the terminal and respiratory bronchioles. | Reduced expansion of lung parenchyma and decrease total lung capacity. |
Pulmonary function test | Decreased FEV1/FVC ratio (<0.7) | Normal FEV1/FVC ratio |
Examples |
|
Property | Squamous cell carcinoma | Adenocarcinoma | Small cell carcinoma | Large cell carcinoma |
|---|---|---|---|---|
Epidemiology |
|
| Highly associated with smoking | |
Etiopathogenesis (Genetic factors) |
|
|
| |
Prognosis and Response to treatment | Good prognosis |
| ||
Metastasis |
| |||
Site of cancer | Central (Hilar region) | Peripheral region | Central (Hilar region) | Peripheral region |
Morphology | Gross graph TD
1["Squamous metaplasia/ Dysplasia"]
2["Carcinoma in situ<br>- Atypical cells may be identified in cytologic smears of sputum or in bronchial lavage fluids or brushings. <br>- Lesion is asymptomatic and undetectable on radiographs. <br>- Last for several years."]
3["Invasive squamous cell carcinoma"]
4["Grows exophytically into the bronchial lumen."]
5["Produces intraluminal mass."]
6["Obstruction of bronchus with further enlargement."]
7["Distal atelectasis and infection."]
8["Penetrate the wall of the bronchus."]
9["Infiltrate along the peribronchial tissue into the adjacent carina or mediastinum."]
10["Tumors grows along a broad front to produce a cauliflower-like parenchymal mass <br>that pushes lung substances ahead of it."]
1 --> 2
2 --> 3
3 --> 4
4 --> 5
5 --> 6
6 --> 7
3 --> 8
8 --> 9
3 --> 10
Microscropic Poorly differentiated
Moderately differentiated forms
Well differentiated forms
| Gross Atypical adenomatous hyperplasia
Adenocarcinoma in situ
Adenocarcinoma
● Microscopic: |
| |
Neuroendocarine markers |
| |||
Paraneoplastic hormones |